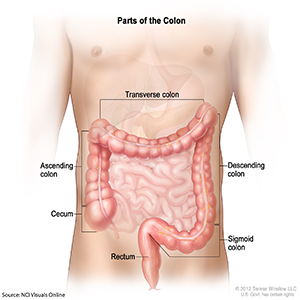
 The National Cancer Institute (NCI) Clinical Proteomic Tumor Analysis Consortium (CPTAC) announces the release of the cancer proteome confirmatory colon study data. The goal of the study is to analyze the proteomes of approximately 100 confirmatory colon tumor patients, which includes tumor and adjacent normal samples, with liquid chromatography-tandem mass spectrometry (LC-MS/MS) global proteomic and phosphoproteomic profiling. The data generated from the confirmatory colon tumor samples are then compared to the data from The Cancer Genome Atlas (TCGA) colorectal proteome study published in Nature. These samples have also undergone genomic characterization. The genomic data will be available from the NCI Genomic Data Commons. Vanderbilt University School of Medicine, which served as a proteomic characterization center (PCC) from 2006 through 2011, and current PCC Pacific Northwest National Laboratory (PNNL) investigated the tumor samples by label-free global proteomic profiling and assayed with tandem mass tag (TMT) 10-plex isobaric labeling quantification. Mass spectrometry analysis was performed using an Orbitrap Fusion Lumos mass spectrometer. The data released include the raw mass spectrometry data files along with the peptide-spectrum match files and protein summary reports. The CPTAC publication embargo for the cancer proteome confirmatory colon study data ends March 19, 2019.
The National Cancer Institute (NCI) Clinical Proteomic Tumor Analysis Consortium (CPTAC) announces the release of the cancer proteome confirmatory colon study data. The goal of the study is to analyze the proteomes of approximately 100 confirmatory colon tumor patients, which includes tumor and adjacent normal samples, with liquid chromatography-tandem mass spectrometry (LC-MS/MS) global proteomic and phosphoproteomic profiling. The data generated from the confirmatory colon tumor samples are then compared to the data from The Cancer Genome Atlas (TCGA) colorectal proteome study published in Nature. These samples have also undergone genomic characterization. The genomic data will be available from the NCI Genomic Data Commons. Vanderbilt University School of Medicine, which served as a proteomic characterization center (PCC) from 2006 through 2011, and current PCC Pacific Northwest National Laboratory (PNNL) investigated the tumor samples by label-free global proteomic profiling and assayed with tandem mass tag (TMT) 10-plex isobaric labeling quantification. Mass spectrometry analysis was performed using an Orbitrap Fusion Lumos mass spectrometer. The data released include the raw mass spectrometry data files along with the peptide-spectrum match files and protein summary reports. The CPTAC publication embargo for the cancer proteome confirmatory colon study data ends March 19, 2019.
To access the cancer proteome confirmatory colon study data sets, visit the CPTAC Data Portal – click here.

