CPTAC researchers out of the University of Michigan have hit another home run. The Nesvizhskii lab, developer of the FragPipe proteomics pipeline, has developed an extension of its MSFragger flagship software to now identify N- and O-linked glycopeptides. The study was recently published in Nature Methods. The new mode, MSFragger-Glyco, allows researchers to have the same ultrafast and sensitive search for glycopeptide spectrum matches, as with the original MSFragger software.
The software, developed by 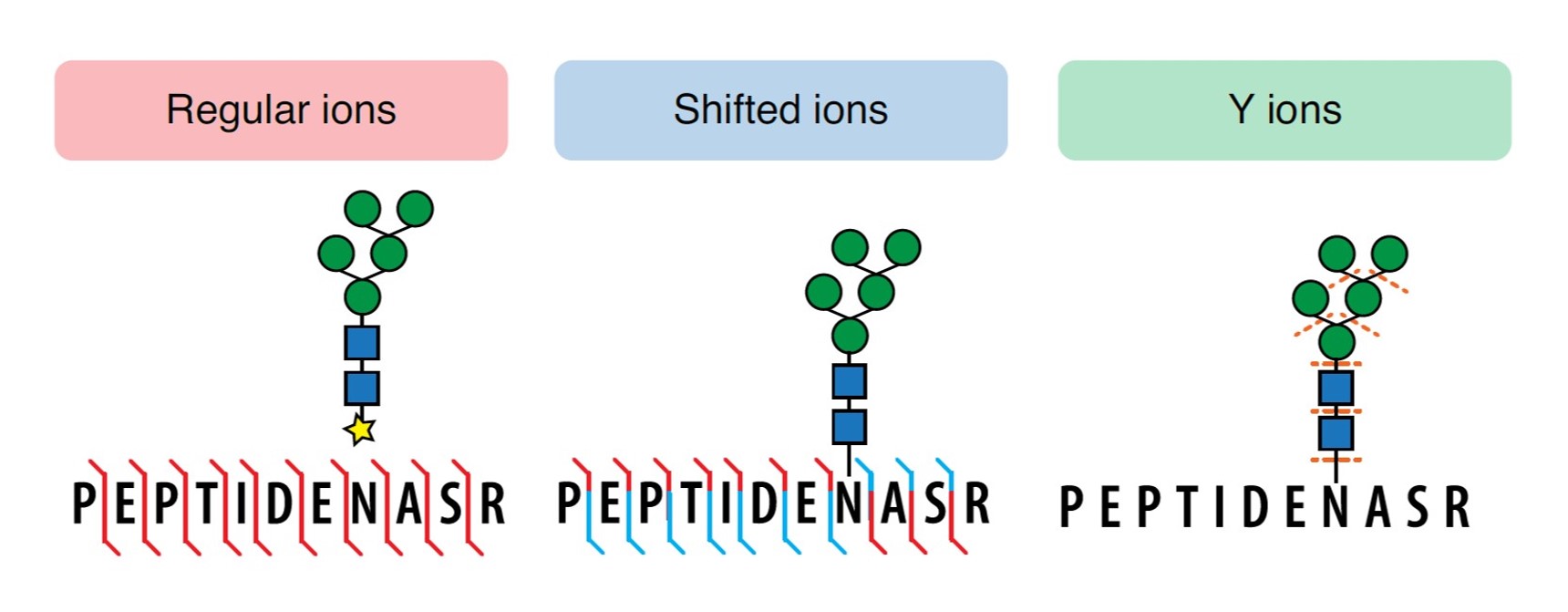 Dr. Dan Polasky (Nesvizhskii lab), allows for spectra to be simultaneously searched against all fragment ion series of interest, ensuring all possible glycoPSM matches while reducing noise from highly unlikely fragments. As proof of concept, the software was applied to several previously published, state-of-the-art glycoproteomics data sets to compare the variation in glycoPSM yield and quality. Reanalysis of N-glycoproteomic data resulted in identification of 80% more glycoPSMs than previously reported. Additionally, the built-in glycan mass offset strategy, along with expanded search criteria, provided a 4- to 6-fold increase in existing O-linked glycoPSMs data sets. With this improved spectral annotation, and speedy processing, the utility of MSFragger-Glyco greatly improves the identification of labile glycan spectra, that can be applied to deciphering complex large-scale N- and O-glycoproteomics.
Dr. Dan Polasky (Nesvizhskii lab), allows for spectra to be simultaneously searched against all fragment ion series of interest, ensuring all possible glycoPSM matches while reducing noise from highly unlikely fragments. As proof of concept, the software was applied to several previously published, state-of-the-art glycoproteomics data sets to compare the variation in glycoPSM yield and quality. Reanalysis of N-glycoproteomic data resulted in identification of 80% more glycoPSMs than previously reported. Additionally, the built-in glycan mass offset strategy, along with expanded search criteria, provided a 4- to 6-fold increase in existing O-linked glycoPSMs data sets. With this improved spectral annotation, and speedy processing, the utility of MSFragger-Glyco greatly improves the identification of labile glycan spectra, that can be applied to deciphering complex large-scale N- and O-glycoproteomics.
Glycosylation of proteins is one of the most ubiquitous post-translational modifications (PTMs), second only to phosphorylation. The glycosylation mechanism moves carbohydrate units on and off specific amino acids to temporarily change the confirmation (or shape) of a protein, allowing it to carry out targeted transport and a myriad of functions within the cellular environment. Cancer cells modify the chemical structure, placement, removal, or addition of these PTM units as a part of carcinogenesis and cancer progression and has been studied closely by many researchers as a complementary way to ameliorate the disease. The broader impact of this work helps cancer researchers further identify even more glyco-modifications that have a strong or direct impact on how tumor development. CPTAC is proud to be on the front lines of cancer research.

