Protein glycosylation, the enzymatic process that attaches glycans or sugar molecules to proteins, plays a crucial role in cancer development processes, such as cell-cell adhesion, cell growth, ligand-receptor binding, and tumor metastasis. Aside from phosphorylation, other protein modifications have not been investigated in large-scale proteomic studies.
In a study today published in Cell Reports, CPTAC investigator 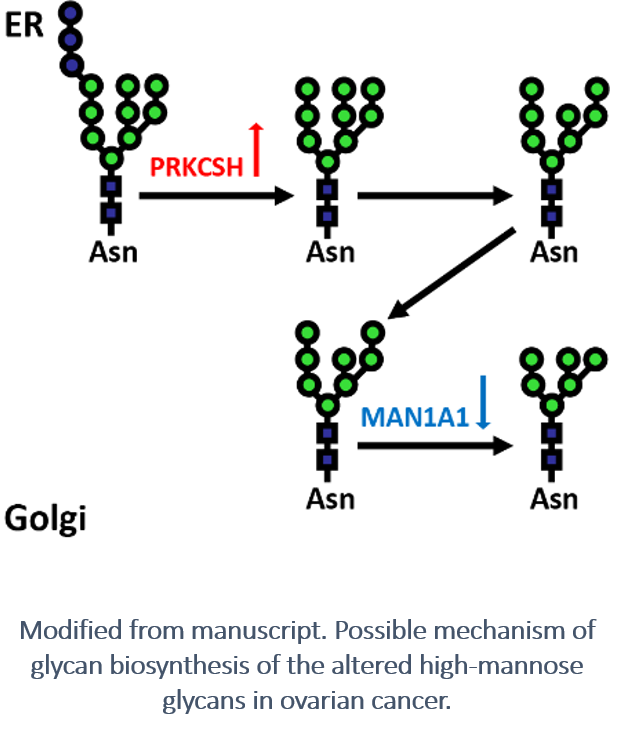 (part of the Proteome Characterization Centers) Dr. Hui Zhang, her team at Johns Hopkins University, and fellow consortium members conducted integrated proteomic and glycoproteomic analyses of 83 prospectively collected high-grade serous ovarian carcinoma (HGSC) tissues and 23 relevant non-tumor tissues.
(part of the Proteome Characterization Centers) Dr. Hui Zhang, her team at Johns Hopkins University, and fellow consortium members conducted integrated proteomic and glycoproteomic analyses of 83 prospectively collected high-grade serous ovarian carcinoma (HGSC) tissues and 23 relevant non-tumor tissues.
This paper is the first-of-its-kind, large-scale protein glycosylation analysis in clinical specimens with annotated clinical metadata. More importantly, understanding the role of altered glycoproteins could provide a foundation for the development of diagnostic and/or therapeutic targets of HGSC.
Cancer antigen 125 (CA125 / MUC16) is a protein often elevated in ovarian cancer. However, the lack of sensitivity and specificity of current CA125 testing in clinical practice points to the need for alternative testing strategies. CPTAC investigators found that glycosylation at two CA125 glycosites detected in all ovarian tumors demonstrated differential expression between tumor and non-tumor samples, suggesting that the degree of protein glycosylation at specific glycosites could be influenced by the pathological status of the tissues.
The findings highlight the need for high quality mass spectrometric data to comprehensively analyze CA125 at each glycosite. Additionally, the finding indicates that the analysis of glycosites of CA125 protein could be used for the detection of ovarian tumors.
Investigators also found that differential expression occurred on specific N-linked glycans that modified the glycoproteins in the tumor samples, in addition to global proteins and glycosites. The up-regulation of PRKCSH (a critical component of the N-linked glycan biosynthesis pathway) and down-regulation of most downstream N-linked glycan biosynthesis enzymes were observed to actively promote the over-expression of high-mannose glycans in cancer tissues.
The findings allowed the investigators to hypothesize that glycosylation biosynthesis pathway may be partially disabled in tumor samples due to the down-regulation of a series of glycosylation enzymes, such as MAN1A1, starting after the trimming of glucoses. This could be an energy-saving mechanism in the tumor to more efficiently manufacture glycoproteins or adapt to environmental constrain.
The glycoproteomic data linking glycoproteins with their extent of protein expression, glycosylation, glycan modifications, and the glycosylation enzymes will improve our understanding of the molecular basis of ovarian cancer.
The comprehensive proteomic and glycoproteomic measurements for the HGSC tumor samples provide a valuable public resource and is made in the CPTAC Data Portal: https://cptac-data-portal.georgetown.edu/cptac/s/S038.

