Prostate cancer screening is typically done by evaluation of levels of prostate-specific antigens (PSA). Unfortunately, its effectiveness in stratifying low risk patients from those with aggressive (AG) prostate cancer is poor. Localized in the Golgi, α (1,6) fucosyltranferase (FUT8), a glycotransferase responsible for catalyzing the addition of fucose onto glycoprotein, has been previously shown to have a role in cell motility and invasiveness, and its over expression has been shown to associated with AG prostate cancer.
CPTAC researchers at John Hopkins University 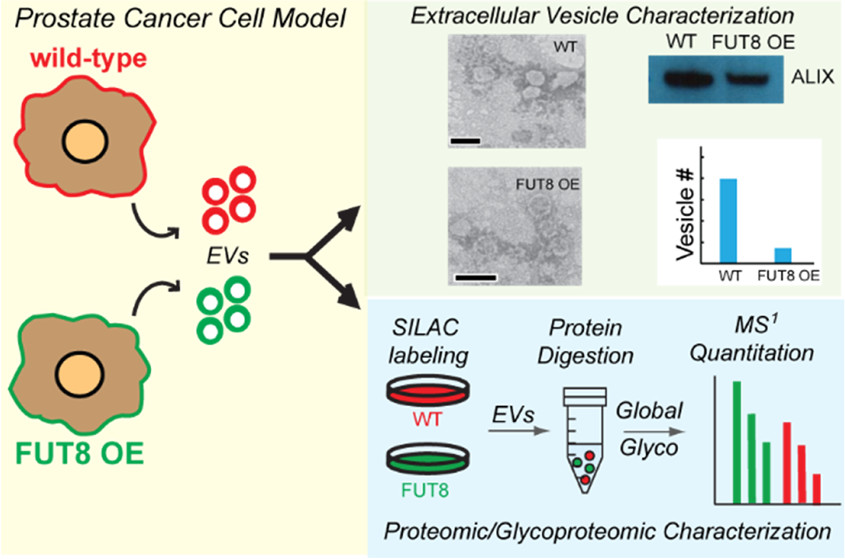 recently released a study examining the impact of altered FUT8 expression on EV biogenesis and subsequent EV protein profiles, comparing aggressive prostate cancer cells to less aggressive ones. Using stable isotope labeling with amino acids in cell culture (SILAC)-based quantitative proteomics paired with nanoparticle tracking analysis (NTA), researchers were able to show that cellular increases in FUT8 expression in cancerous cells could reduce the overall number of secreted EVs, while increasing the abundance of cell motility and metastasis-associated proteins within them. Interestingly, alterations of FUT8 was founds to have no impact on the average size of the vesicles. Additionally, evaluation of the EV cargo revealed proteins associated with an AG prostate cancer phenotype elevated in FUT8-transformed EVs, while capturing aberrant activity of the cells endocytic-related machinery.
recently released a study examining the impact of altered FUT8 expression on EV biogenesis and subsequent EV protein profiles, comparing aggressive prostate cancer cells to less aggressive ones. Using stable isotope labeling with amino acids in cell culture (SILAC)-based quantitative proteomics paired with nanoparticle tracking analysis (NTA), researchers were able to show that cellular increases in FUT8 expression in cancerous cells could reduce the overall number of secreted EVs, while increasing the abundance of cell motility and metastasis-associated proteins within them. Interestingly, alterations of FUT8 was founds to have no impact on the average size of the vesicles. Additionally, evaluation of the EV cargo revealed proteins associated with an AG prostate cancer phenotype elevated in FUT8-transformed EVs, while capturing aberrant activity of the cells endocytic-related machinery.
This study establishes evidence of how altered cellular glycosylation enzyme expression impacts EV vesicle abundance and the protein content of EVs secreted from prostate cancer cells via dysregulation of the cell’s endocytic pathway. It also shows how differences in EV protein cargo, including glycosite occupancy and glycosylation patterns, may contribute to prostate carcinogenesis – making a case for EV-based diagnostics that incorporate glycosylation information for prostate cancer screening. Although a frequently overlooked contribution to disease pathology, this study provides confirmation of glycosylation’s crucial part in disease progression and CPTAC researchers are at the forefront. This study was partially funded by NCI grant U24CA210985 and U01CA152813.

