The National Cancer Institute's (NCI) Clinical Proteomic Technologies for Cancer (CPTC) initiative at the National Institutes of Health has entered into a memorandum of understanding (MOU) with the Korea Institute of Science and Technology (KIST). This MOU promotes proteomic technology optimization and standards implementation in large-scale international programs. The MOU framework will enable and encourage the sharing of knowledge and support the formation of research teams to solve complex problems in the area of each organization's mission.
Achievements in science and technology now require a higher level of integration, particularly in the development of interdisciplinary research teams. The programmatic strengths of both the NCI and KIST in advancing the overall study of clinical proteomics offer a unique opportunity for both organizations to join their efforts in order to accelerate proteomics technology development and application in clinical settings on a global scale. A specific area of interest is the development of quantitative clinical proteomic workflows.
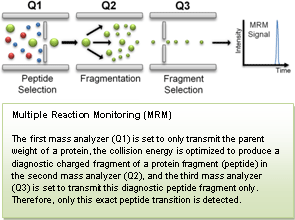 NCI's CPTC, through its Clinical Proteomic Technology Assessment for Cancer (CPTAC) Network, recently conducted a quantitative round-robin study on multiple reaction monitoring (MRM) mass spectrometry. This landmark study, published in Nature Biotechnology(1), describes a multi-laboratory study to assess the reproducibility, recovery, linear dynamic range, and limits of detection and quantification of multiplexed, MRM-based assays. CPTAC Network investigators demonstrated that using common materials and standardized protocols, these assays can be highly reproducible within and across laboratories and instrument platforms.
NCI's CPTC, through its Clinical Proteomic Technology Assessment for Cancer (CPTAC) Network, recently conducted a quantitative round-robin study on multiple reaction monitoring (MRM) mass spectrometry. This landmark study, published in Nature Biotechnology(1), describes a multi-laboratory study to assess the reproducibility, recovery, linear dynamic range, and limits of detection and quantification of multiplexed, MRM-based assays. CPTAC Network investigators demonstrated that using common materials and standardized protocols, these assays can be highly reproducible within and across laboratories and instrument platforms.
In addition, the CPTAC Network, working with the U.S. Food and Drug Administration (FDA), recently published mock 510(k) pre-submissions for protein-based platforms: a multiplex immunoaffinity mass spectrometry platform for protein quantification, and an immunological array platform quantifying glycoprotein isoforms (2,3). These submissions provided a mutually beneficial way for members of the proteomics and regulatory communities to identify the analytical issues that the field should address when developing protein-based multiplex clinical assays.
The newly signed MOU between KIST and the NCI will build on these recent accomplishments of the CPTAC Network and help in the dissemination and testing of new proteomics workflows.
Background Information
The NCI CPTC links clinicians with technologists and cancer biologists to accelerate the optimization and standardization of proteomic technologies for the detection of cancer-relevant proteins in clinical specimens. Specifically, it is addressing the limitations and challenges in applying clinical proteomics to alleviate the cancer burden through the development of performance standards, Standard Operating Procedures (SOPs), high-quality reagents, and access to historical analytical reference data. Through this process, CPTC fosters the building of an integrated foundation that is systematically advancing the application of protein science to accelerate discovery and clinical research in cancer.
The Functional Proteomics Center (FPC) was created at KIST to establish an infrastructure of proteomics core technology in Korea with a mission of identifying novel biomarkers and therapeutic target proteins for human diseases. The FPC is one of 21C Frontier Research and Development Initiatives of the Korean Ministry of Education, Science and Technology. The research teams in the program focus on technology development for proteome separation and identification, as well as on proteomic analysis of samples from disease models and appropriate patients, which will be followed by studies on protein networks and disease mechanisms. KIST leads in efforts to build a science and technology-based society in South Korea through supporting national aspirations for science and technology, and also leads the fusion of research in frontier and emerging technologies.
References
- Addona, T., Abbatiello, S.E., Skates, S.J., Bunk, D.M., Schilling, B., Spiegelman, C.H., Zimmerman, L.J., Ham, A-J.L., Keshishian, H., Hall, S.C., Allen, S., Anderson, N.L., Blackman, R.K., Borchers, C.H., Buck, C., Cardasis, H.L., Cusack, M.P., Dodder, N.G., Gibson, B.W., Held, J.M., Hiltke, T., Jackson, A., Johansen, E.B., Kinsinger, C.R., Li, J., Mani, D.R., Mesri, M., Neubert, T.A., Niles, R.K., Paulovich, A.G., Pulsipher, T.C., Rodriguez, H., Rudnick, P.A., Smith, D., Tabb, D.L., Tegeler, T.J., Variyath, A.M., Vega-Montoto, L.J., Wahlander, A., Waldemarson, S., Wang, M., Whiteaker, J.R., Fisher, S.J., Liebler, D.C., Regnier, F.E., Tempst, P., Carr, S.A., CPTAC Network. (2009) Multi-site Assessment of the Precision and Reproducibility of Multiple Reaction Monitoring-based Measurements of Proteins in Plasma. Nature Biotechnology. Jul;27(7):633-644.
- Rodriguez, H., Težak, Z., Mesri, M., Carr, S.A., Liebler, D.C., Fisher, S.J., Tempst, P., Hiltke, T., Kessler, L.G., Kinsinger, C.R., Philip, R., Ransohoff, D.F., Skates, S.J., Regnier, F.E., Anderson, N.L., Mansfield, E., on behalf of the Workshop Participants. (2010) Analytical Validation of Protein-Based Multiplex Assays: A Workshop Report by the NCI-FDA Interagency Oncology Task Force on Molecular Diagnostics. Clinical Chemistry. Jan;56(2):237-243.
- Regnier, F.E., Skates, S.J., Mesri, M., Rodriguez, H., Težak, Z., Kondratovich, M.V., Alterman, M.A., Levin, J.D., Roscoe, D., Reilly, E., Callaghan, J., Kelm, K., Brown, D., Philip, R., Carr, S.A., Liebler, D.C., Fisher, S.J., Temps, P., Hiltke, T., Kessler, L.G., Kinsinger, C.R., Ransohoff, D.F., Mansfield, E., Anderson, N.L. (2010) Protein-Based Multiplex Assays: Mock Presubmissions to the U.S. Food and Drug Administration. Clinical Chemistry. Jan;56(2):165-171.

